 A Theory of Stuttering
A Theory of Stuttering
My theory of stuttering assumes a misallocation of attention during speech to be the primary cause for the development of stuttering. But what causes that cause? Why do stutterers tend to allocate their attention inappropriately when speaking? One important factor has already been discussed extensively in Section 2.6: higher demands of speech planning—more precisely, a new quality of demands—when children start forming sentences. A second factor, particularly if stuttering onsets later in childhood or in adulthood, could be experiences that strain communication and make a person plan speech overly carefully or worry about the listeners’ reactions.
However, the question arises as to why some individuals are more sensitive to such factors than the majority, who don’t develop stuttering. Empirical findings point in two directions: (1) a general deficit in the automatic regulation of attention, and (2) a deficit in central auditory processing, which may impair the automatic control of auditory attention.
In general, the prevalence of attention-deficit hyperactivity disorder (ADHD) in school-age children is 3–6% (Donaher, Healey, and Soffer, 2013). Similar to persistent stuttering, ADHD is more prevalent in males than in females (Ramtekkar et al., 2010). Data on the prevalence of ADHD in children who stutter range from 4% to 26% (Healey & Reid, 2003; Alm, 2014). In a study with 204 stuttering children between 5 and 18 years of age, Walsh et al. (2025) found 17.2% with diagnosed ADHD. Donaher and Richels (2012) found 58%, and Walsh, Tichenor, & Gerwin (2025) found over 40% of stuttering children without ADHD diagnosis to show symptoms of ADHD that warrant further examination (read more).
Alm (2014) and Wagovich, Anderson, and Hill (2020), for instance, have given overviews of the literature concerning stuttering and attention. Findings suggest that stutterers tend to be less efficient in attention regulation and less able to divide their attention under dual task conditions . Eggers, De Nil, and Van den Bergh (2012) concluded from their findings that the orienting network that plays an important role in the allocation of attention appears to be less efficient in children who stutter. Kaganovich, Hampton Wray, and Weber-Fox (2010) concluded from an examination of auditory processing in stuttering preschoolers that stuttering may be associated with less efficient attention allocation.
In an EEG study of attention-related brain function, Ratcliff-Baird (2001) found “strong similarities in the EEG, morphology, and behavior of stutterers and individuals with ADHD”. Karrass et al. (2006) asked parents of 65 stuttering and 56 non-stuttering preschool children to complete a questionnaire about the children’s emotional reactivity and regulation. The findings indicated that, when compared to controls, stuttering children were more reactive, less able to regulate their emotions, and less able to flexibly control their attention.
A special combination of deficient attention regulation, on the one hand, and hyperactivity, on the other hand, seems to play a role in childhood stuttering: a tendency to overly focus on a target, on action (on the cost of perception), or to shift attention prematurely from the current step of a sensorimotor sequence to the next one. Jansson-Verkasalo et al. (2012) found that stuttering children, as a group, showed more premature responses than age-matched normally fluent controls in a task aimed at measuring auditory attention. Adults who stutter, as a group, compared to controls, showed more false alarms in a syllable recognition task (Bosshardt, 1993) and increased stop-signal reaction times in two tasks that did not rely on speech production (Markett et al., 2016). Likewise, stuttering children exhibited more false alarms and premature responses compared to controls in Go/No Go paradigms (Chou, 2014; Eggers, De Nil, & Van den Bergh, 2013).
It is of interest in our context that ADHD, despite its label as an attention deficit disorder, is recently more considered an attention allocation deficit, rather than high distractibility or the disability to concentrate. I don’t believe that stutterers are generally inattentive or more distractible than non-stutterers. To the contrary, I think that they often overly focus on a target; during speech, for example, on the thoughts or emotions they want to express, on sentence formulation, or on the avoidance of stuttering, with the effect that other important information is poorly processed.
Empirical findings support this view. Using a norm-referenced parent-report questionnaire, Anderson et al. (2003) found stuttering preschoolers (mean age: 4 years) to be less distractible as a group, compared to controls. Clark et al. (2015) examined the distractibility of stuttering preschoolers and controls and found that stuttering boys, as a group, were less distractible than both, stuttering and non-stuttering girls. For the stuttering boys, less distractibility, i.e., greater (!) selective attention was associated with more speech-language dissociations (imbalances among subcomponents of speech-language planning and production). Likewise, Singer, Walden, and Jones (2019) found children who persisted in stuttering less distractible (based on parent reports) than children who recovered. A propensity to focus attention on only one target and to ignore everything else is beneficial in many respects (read more), but seems to raise the risk of stuttering.
An additional factor may be responsible for the different prevalence of stuttering in males and females. Foundas et al. (2004) examined the ability of adult stutterers and controls to focus on one ear in a dichotic verbal listening task. Male stutterers, as a group, did not differ from controls (left-handed men who stutter could shift attention to the left or right ear even better than any other group). Right-handed women who stutter, by contrast, made the most errors and were relatively unable to focus their attention selectively on the left or right ear (left-handed women who stutter couldn’t be recruited). The results suggest that there may be two subtypes regarding the kind of attention deficit. One group (the majority of the men who stutter?) is well able to control their auditory attention deliberately when actively listening (which doesn’t imply appropriate automatic attention allocation during speech). The other group (the majority of the women who stutter?) has a basic deficit in the control of auditory attention, possibly inherent. The latter is consistent with the fact that the percentage of women in familial stuttering is much greater than in extra-familial stuttering (Drayna, Kilshaw, & Kelly, 1999); that is, genes seem to play a greater role in persistent stuttering in females than in males.
Using fMRI, Chang et al. (2018) investigated the resting state connectivity within several intrinsic connectivity networks in stuttering children, children who had recovered from stuttering, and normally fluent controls. In their Discussion (Section 4.5), they summarize their findings as follows: “… persistent stuttering seems to be associated with connectivity differences that primarily involve DMN and its connectivity with attention and executive control networks. Alternatively, recovered children exhibit similar connectivity deficits to persistent children in many respects, but normalized connectivity involving DMN and attention and executive control networks may help them recover.” (DMN = default mode network, a set of brain regions that are deactivated during goal-directed tasks; functionally, the DMN is associated with inwardly focused attention, e.g., introspection and daydreaming). (read more).
Doneva, Davis, and Cavenagh (2018) examined the attentional performance of adult stutterers. They used the Test of Everyday Attention (TEA), which comprises eight subtests that pose different demands on sustained attention, selective attention, attention switching, and divided attention. The stutterers performed worse on tasks tapping into visual selective and divided attentional resources. The former corresponds to the surprising finding of reduced functional connectivity in the visual network found by Chang et al. (2018) in stuttering children; the latter confirms the results of several earlier studies (see above). There was further a trend for stuttering to be associated with poorer performance on two subtests measuring attentional switching and one tapping into auditory selective attention.
Compilation of more than 50 studies concerning auditory processing and stuttering in chronological order, with a short abstract (methods and results) of each study.
Numerous studies have been done to examine hearing and central auditory processing in stutterers. Both children and adults who stutter, as groups, exhibited deviant auditory processing of verbal stimuli or performed poorer than controls (Andrade et al., 2008; Beal et al., 2010, 2011; Blood & Blood, 1984a; Blood, 1996; Chang et al., 2009; Corbera et al., 2005; Halag-Milo et al., 2016; Hall & Jerger, 1978; Lu et al., 2016; Neef et al., 2012; Tahaei et al., 2014).
Some findings suggest that the auditory feedback of speech is either poorer processed or less involved in speech control in stutterers, compared with normally fluent speakers. For instance, Cai et al. (2012, 2014a) and Loucks, Chon, and Han (2012) found weaker-than-normal compensatory responses to unexpected alterations of auditory feedback in adults who stutter.
More importantly, stutterers differ from normally fluent speakers even in the processing of non-speech auditory stimuli (e.g., Chang et al., 2009; Dietrich, Barry, & Parker, 1995; Hampton & Weber-Fox, 2009; Howell et al., 2000; Howell, Davis, & Williams, 2006; Prestes et al., 2017). Some results suggest an overall higher sensitivity to acoustic stimuli: MacCulloch and Eaton (1971) and Brown, Sambrooks, and MacCulloch (1975) found that stuttering children, as a group, had a reduced mean threshold for auditory discomfort compared to controls.
Kikuchi et al. (2011), using click sounds for stimuli in an MEG study, found adults who stutter to have a less effective auditory gating on the left brain hemisphere (i.e., they are less able to suppress the processing of redundant acoustic input) and an expanded tonotopic map on the right hemisphere, suggesting a higher sensitivity to nonverbal acoustic stimuli. Evidence for reduced auditory gating was also found by Saltuklaroglu et al. (2017) in an EEG study.
Gregory et al. (2925) found stuttering children (7 – 11 years of age) to have longer P3 latencies than controls in the auditory evoked potential in response to pure tones, which may “indicate that stutterers perceive acoustic parameters with less precision and with longer reaction times” (p. 8). The P3 amplitude was smaller in the stuttering children, suggesting that they “require a greater neuron activation to differentiate and interpret auditory stimuli” (p. 8). The authors conclude from these findings “that the physiological processes devoted to attention and working memory are less robust in children who stutter.” (p. .8) The results of behavioral tests in the study showed that stuttering children “have difficulty in temporal resolution, figure-ground discrimination, binaural separation, duration discrimination, temporal ordering, and recognition of verbal sounds in monotic and dichotic listening.” (p. 8)
Some researchers have pointed to the brainstem as a possible source for an auditory processing deficit in stutterers, e.g., Blood and Blood (1984b), Khedr et al. (2000), and Kramer, Green, and Guitar (1987). Tahaei et al. (2014) found in adult stutterers, compared to controls, longer latencies for the onset and offset peaks in auditory brainstem response to the presented syllable /da/ and correlations between some of these latencies and stuttering severity. They conclude that the neural response to rapid acoustic transients was less synchronous in the stuttering participants.
Tahaei et al. (2014) speculated that their results may either be linked to timing disturbances in the auditory pathways, resulting in asynchronous transmission of auditory afferent information, or to top-down influences from the auditory cortex (memory, language experience, and attention) through the corticofugal system. Particularly the latter possibility is interesting in the context of the present theory.
Asal and Abdou (2014) investigated central auditory processing in stuttering and non-stuttering school-age children by means of several standardized tests. Interestingly, the stuttering children performed poorer than controls in the more complicated tasks, which place demands on auditory attention and working memory. The authors conclude that brain stem integrity is intact in the stuttering children, but they have an auditory processing deficit in the left hemisphere.
Rousey, Goetzinger, and Dirks (1959) reported stutterers to have difficulty with sound localization (I have this problem myself too). Salmelin et al. (1998) found the basic functional organization (interhemispheric balance) of the auditory cortices in stutterers to differ from that in nonstutterers—the sensitivity of the brain hemispheres to the side of stimulation was different. The authors concluded: “The interhemispheric balance is more unstable in stutterers than in fluent speakers and is readily disturbed by increase in work load. Such disturbance may cause transient, unpredictable abnormalities in auditory perception, which could well initiate stuttering and facilitate the emergence of other, related disturbances in the control of speech.” (p. 2229)
In summary, deficits in attention regulation, particularly a tendency toward hyperactivity or impulsivity, and a subtle anomaly in central auditory processing seem to contribute to a predisposition for stuttering.
Deficits in the control of auditory attention and in auditory gating may be two sides of the same coin. Auditory gating suppresses the processing of redundant acoustic information (simply said, of noise); thus, it is basic for the control of auditory attention. If auditory gating doesn’t work well, the person must compensate for that deficit on a higher stage, on which auditory attention can be controlled by the will. Poor auditory gating may frequently result in acoustic over-stimulation; consequently, affected children may soon get into the habit of turning their attention away from the auditory channel to prevent auditory discomfort. Only when actively listening they may direct sufficient attention to the auditory channel, but not in other situations, e.g., when speaking (read more).
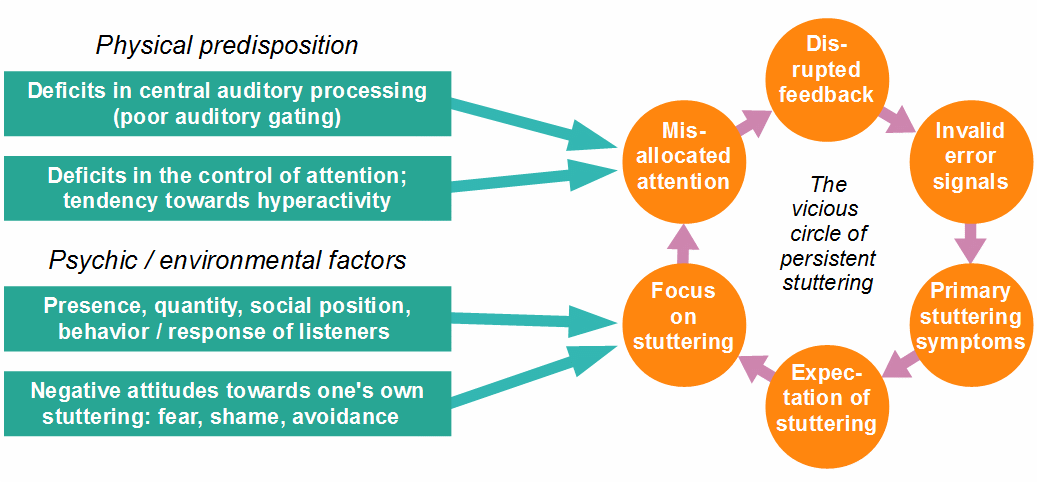
Figure 11: Factors contributing to a predisposition for stuttering, and factors that influence the frequency and severity of symptoms (the circle has been rotated here, compared to Fig. 10, only for reasons of depiction). Note that the main interface between the vicious circle and all influencing factors is the misallocation of attention.
Riley and Riley (2000) found that approximately a quarter of their stuttering children (26%) met their criteria for the presence of a significant attentional deficit. Moreover, among several candidate predictor variables that they examined, the presence of pre-treatment attentional problems was found to be the single best predictor of poor treatment outcome at follow-up; that is, children in this sample who stuttered and had poor attention were significantly less likely than those with adequate attention to have a positive treatment outcome at 24-48 months post-treatment, regardless of factors such as pre-treatment stuttering severity.
Hamilton et al. (2008) found lower fractional anisotropy (a deficit in the microstructure of white matter in the brain) in the superior longitudinal fasciculus in children and adolescents with ADHD, compared to age-matched controls without ADHD. Interestingly, lower fractional anisotropy in the superior longitudinal fasciculus was also found in children, adolescents, and adults who stutter e.g., Sommer et al., 2002; Chow & Chang, 2017).
(return)
Bajaj ( 2007) wrote: “Interestingly, results point to greater ‘costs’ of divided attention among people who stutter for nonverbal measures. Studies in finger tapping suggest consistently that, in contrast to typically fluent individuals, people who stutter lag in their finger tapping performance under concurrent conditions, both verbal (Greiner, Fitzgerald, & Cooke, 1986; Sussman, 1982) and nonverbal (Smits-Bandstra, De Nil, & Rochon, 2006; people who stutter also exhibited lower color recognition accuracy). Notably, significantly lower finger tapping rates under verbally demanding concurrent tasks were also reported among children who stutter (Brutten & Trotter, 1986). Cognitive–linguistic costs associated with concurrent verbal loads are evident as well. Bosshardt, Ballmer, and De Nil (2002) and De Nil and Bosshardt’s (2000) findings suggest that concurrent conditions elicited less sophisticated linguistic performance, such as fewer propositions and reduced rhyming accuracy, among people who stutter than typically fluent individuals.” (p. 228f.)
Maxfield et al. (2016) investigated speech production in an attention-demanding dual-task condition. Adults who stutter and normally fluent controls named pictures that were overlaid with printed distracting words and, at the same time, monitored acoustic stimuli: low tones versus high tones, presented in several patterns. During the tasks, the P3 component of the event-related brain potential was recorded (P3 indicates a late stage of processing). The authors detected a P3 effect in the controls in all dual task conditions. In the stuttering participants, by contrast, the P3 effect was attenuated or undetectable if the tone was presented timely close to the picture. The researchers concluded that, for adults who stutter, the availability of attentional resources for secondary task processing was reduced.
(return)
Harald Euler from the University of Frankfurt/Main, an expert in evolutionary psychology, said the following: If stuttering is genetically caused, and if stutterers are handicapped in social life and professional career and, therefore, have difficulty finding a partner, such that their reproductive success is lower on average than that of the normal fluent population—if all that has been true since the time when humans became able to talk, then stuttering should have gone extinct. But stuttering hasn’t gone extinct; therefore, stutterers must have something (special abilities?) that has compensated for their handicap. So far Euler. His argument is somewhat social Darwinist, but worth considering.
(return)
Brewer et al. (2016) wrote: “A person with an APD typically has difficulty understanding speech in background noise despite having normal pure-tone hearing sensitivity. The estimated prevalence of APD may be as high as 10% in the pediatric population, yet the causes are unknown and have not been explored by molecular or genetic approaches.” They determined the heritability of frequency and temporal resolution for auditory signals and speech recognition in noise in 96 identical or fraternal twin pairs, aged 6-11 years. The study provided evidence of significant heritability, ranging from 0.32 to 0.74, for individual measures of diverse non-speech-based auditory processing skills that are crucial for understanding spoken language. It might be very interesting to compare the genetic variants associated with APD and those associated with developmental stuttering.
(return)
A study by Arcuri, Schiefer, and Azevedo (2017) provides new suggestions to an auditory processing disorder at least in a subgroup of stutterers. The authors found that the suppression of otoacoustic emissions was present in only 7 of 15 stuttering participants, but in 14 of 15 controls. The chance of an individual in the stuttering group to not exhibit a suppression effect was 16 times that of an individual in the control group.
A study by Arcuri, Schiefer, and Azevedo (2017) provides new evidence of an auditory processing deficit in at least a subgroup of stutterers. The authors found that the suppression of otoacoustic emissions was present in only 7 of 15 stuttering participants, but in 14 of 15 controls. The chance for an individual in the stuttering group to not exhibit a suppression effect was 16 times that for an individual in the control group.
Otoacoustic emissions (OAEs) result from the resonance of outer hair cells in the cochlea, which amplifies incoming acoustic stimuli. In doing so, it supports auditory perception and the control of auditory attention. OAEs arise automatically by resonance and are suppressed if irrelevant acoustic input, e.g., noise, is not to be processed.
The suppression takes place through the olivocochlear tracts connecting both ears with one another and with the corticofugal tract, through which the cerebrum can control cochlear activity. In the cochlea, outer hair cells are tonotopically organized, such that their resonance (OAE) is frequency-dependent, and OAE suppression can also affect specific frequencies. The functioning of OAE suppression has not yet been entirely understood, but there seem to be two ways; one of them is attention-dependent, the other one is not.
The latter, attention-independent way is that the ear at which an acoustic signal first or stronger arrives inhibits the OAEs to the signal through the olivocochlear tracts. This simple mechanism supports the quick localization of sound sources and the suppression of background noise that reaches both ears equally (by mutual OAE suppression). That is, the mechanism supports quick adaptation to an acoustic situation and provides the basis for the control of selective auditory attention (see, e.g., Markevych et al., 2011; Srinivasan et al., 2012; Suga et al., 2000).
The other, attention-dependent way of OAE suppression is controlled by the cerebrum through the corticofugal pathway. It serves for the selective suppression of redundant, disturbing, or distracting auditory input. This mechanism depends on selective attention because the brain must first know the target of processing (e.g., a certain person’s voice) and which sounds or sound components (frequencies) are redundant (e.g., noise or music in the background). Selective OAE suppression supports the adjustment and maintenance of auditory attention.
Suggestions that OAE suppression may not work well in some stutterers also come from reports that stutterers find their own voice to be loud, booming, or unpleasant (see, e.g., this report in an online forum). Possibly, OAEs to redundant low frequencies are not sufficiently suppressed in such cases. Further findings may be applicable to impaired OAE suppression, for instance, stutterers’ difficulty with sound localization (Rousey, Goetzinger, & Dirks, 1959) and their reduced mean threshold for auditory discomfort (MacCulloch & Eaton, 1971; Brown, Sambrooks, & MacCulloch, 1975).
(return)
Chang et al. (2018) write that, in the course of child development, the connectivity between the default mode network (DMN) and other intrinsic connectivity networks (ICNs) becomes lower in favor of behavioral control by “task positive” networks. Looking at the figures in the paper, particularly Figure 6, one can see many blue lines between DMN and other ICNs, indicating lower connectivity. Apparently, the segregation between networks is generally more (prematurely) progressed in children who stutter, which may interfere with a gradual, balanced integration of attention, perception, and motor control (I think the important role of attention is sometimes neglected in the common term ‘sensorimotor integration’).
Component 5 (see Fig. 3 in the paper), which predicts persistence in stuttering, shows many instances of decreased connectivity, but also some of hyperconnectivity, particularly between frontoparietal network (FPN) and DMN and between dorsal attention network (DAN) and DMN. This may indicate an imbalance in attention control, possibly too close a link between executive control and top-down attention (focused on targets), at the cost of feedback processing, which may be more based on bottom-up attention.
A further interesting finding is the hyperconnectivity between DMN and Ventral Attention Network (VAN) in stuttering children (see Fig. 5 in the study). Given that functional connectivity means that the brain areas tend to be activated or deactivated synchronously, and that DMN is usually deactivated in goal-directed tasks, then the hyperconnectivity between DMN and VAN suggests that VAN tends to be deactivated in goal-directed tasks in stuttering children. That’s a problem because VAN is responsible for bottom-up attention, which is needed for automatic self-monitoring and feedback processing.
(return)