 A Theory of Stuttering
A Theory of Stuttering
When Sommer et al. (2002) published their findings of white matter deficits in the brain in adult stutterers, they hoped they had discovered the cause of stuttering: disturbed signal transmission between speech-relevant brain areas. Specifically, they found reduced fractional anisotropy in the left superior longitudinal fasciculus (SLF), a white matter tract interconnecting the temporoparietal region with lateral pre-motor and motor areas involved in speech control—roughly, Wernicke’s and Broca’s areas (see also Büchel & Sommer, 2004). Similar results were obtained in further studies in adults (Cai et al., 2014b: Connally et al., 2014; Cykowski et al., 2010; Watkins et al., 2008) and children who stutter (Chang et al., 2008, 2015; Chow & Chang, 2017).
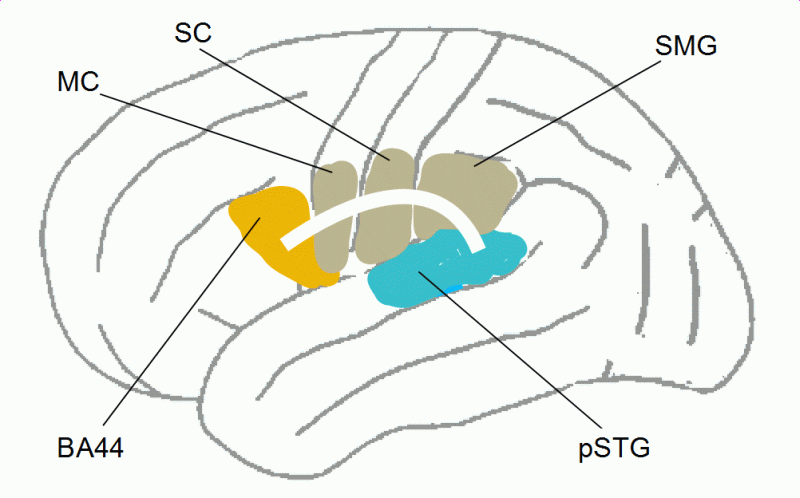
Figure 15: Charting of the superior longitudinal fasciculus (SLF) on the left brain hemisphere, connecting the posterior superior temporal cortex, the supramarginal gyrus (SMG) of the inferior parietal cortex, and the posterior part of Broca’s area (pars opercularis, BA44) (read more). MC = motor cortex, SC = somatosensory cortex (both involved in speech motor control, among others).
Reduced fractional anisotropy (FA) in white matter has mainly been interpreted as reduced density or reduced structural integrity of the nerve fibers, and particularly as a lower degree of myelination. Indeed, FA can also be reduced because of crossing fibers, a smaller number of fibers, or an uneven structure of the bundles, but I will leave those possibilities aside here because such relatively invariable structural features can hardly cause a disorder as variable as stuttering.
The reduced FA found in the SLF in stutterers suggests a lower degree of myelination, that is, of maturation of the nerve fibers. Nerve fibers (axons) are not a kind of wire between neurons, but the axon is the largest part of a neuron. A neuron’s activity is also the activity of its axon. Knowing that is important to understand the mechanism of myelination and to interpret deficits in white matter structure.
Myelination proceeds in the brain as follows: Oligodendrocytes, a sort of glia cells, wind themselves in layers around the axon. The myelin sheath enables a ‘saltatory’ conduction of impulses, in contrast to the continuous conduction in an axon without myelin sheath. This increases the speed at which impulses propagate along the axon: in a myelinated axon, transmission is up to 16-times faster.
Myelination takes place at different times and at different speeds in the brain. Within the speech network, it proceeds mainly in childhood and adolescence when language and speech abilities develop. For instance, Brauer (2009) found that FA in the SLF was significantly reduced in seven-year-old normally fluent children compared to adults. Therefore, the reduced fractional anisotropy within the speech network in stutterers may result from a delay in myelination. However, the findings themselves don’t tell us whether they are causal for stuttering or merely a consequence or concomitant of the disorder. To answer this question, let us consider how myelination is controlled.
Bengtsson et al. (2005) investigated the effect of piano practicing in childhood, adolescence, and adulthood on white matter. They found a positive correlation between practice and FA in various brain regions involved in piano playing. Scholz et al. (2009) conducted a longitudinal study in which participants learned a new visuomotor skill: juggling. The training group and an untrained control group were scanned before and after a six-week training period. In the trained group, FA was increased in white matter underlying cortex regions that were involved in the control of juggling.
A similar study was done by Keller and Just (2009), who investigated whether 100 hours of intensive reading exercises affected the white matter structure of 8- to 10-year-old poor readers. The intensive practice resulted in increased FA in a brain region where fractional anisotropy was lower on average in the poor readers compared with good readers. The increase in FA was correlated with improved reading and a decrease in radial diffusivity but not with a change in axial diffusivity, suggesting increased myelination.
Takeuchi et al. (2010) had young adults practice memorizing the spatial and temporal order of visually presented stimuli, 25 minutes a day over two months. The amount of training was correlated with an increase in FA in white matter tracts critical for working memory. Together, these findings suggest a correlation between activity over time and myelination: the more frequently fibers are active, the better they become myelinated, similar to muscle that becomes stronger through training.
This has been confirmed by molecular biology. Wake, Lee, and Fields (2011), with axons of mice in culture, observed a mechanism that promotes the myelination of electrically active axons, in which the axon’s electrical activity provides a signal to oligodendrocytes to produce the basic protein for myelin. The more frequently an axon is active, that is, the more often the neuron ‘fires’, the better it becomes myelinated (see also Fields, 2010; Zatorre, Fields, & Johansen-Berg, 2012) (read more).
What is the function of the correlation between axonal activity and myelination? As mentioned above, myelination accelerates impulse conduction along the axon. The benefit is not so much that the organism quicker reacts, but that the fastest neuronal network can suppress competitive networks and determine the organism’s behavior. This makes sense from the viewpoint of evolution and anables non-cognitive learning (read more).
Let us return to the question of why some parts of the left SLF in stutterers are less myelinated. If frequently active fibers are preferentially myelinated, then it is not surprising that fibers that were less active in the past are less myelinated. The reduced FA in the left SLF in stutterers may therefore result from long-term reduced activity of these fibers. Reduced activiation of left SLF fibers may be related to the under-activation in the posterior superior temporal cortex (Wernicke’s area) found in stutterers as compared with normally fluent speakers (see Table 1), which is caused by insufficient attention to auditory feedback when speaking.
We can thus summarize: stutterers pay too little attention to auditory feedback when speaking; that’s why left-hemispheric secondary auditory areas are under-activated, with the consequence that the fibers interconnecting this brain region with the left inferior frontal cortex are less active, and long-standing under-activation of the fibers results in a delay in myelination. As in the above-mentioned experiments by Scholz et al., (2009), Keller and Just (2009), and Takeuchi et al. (2010), practicing listening to one’s voice while speaking should not only reduce stuttering but also, with time, improve the myelination in the left SLF.
In brain research, fractional anisotropy (FA) is a measure describing the degree of anisotropy in the diffusion of water molecules. A value of zero means that diffusion is isotropic; that is, it is equal in all spatial directions. In and around nerve fibers, diffusion is anisotropic, namely, lower orthogonal to the direction of the fibers and/or higher in fiber direction. Tendentially, anisotropy is the higher the better the fibers are coated with myelin, although some other factors also influence FA, for instance, crossing fibers.
However, radial diffusivity (perpendicular to fiber direction) is clearly correlated to myelination: the lower the radial diffusivity, the thicker the myelin sheath. The relation of axial diffusivity (in fiber direction), to myelination or to the maturation of the fibers in general is still a matter of debate (see, e.g., Alexander et al., 2007; Bartzokis et al., 2012; Mädler et al., 2008; Uda et al., 2015; Wei et al., 2013).
(return)
The exact anatomy of the dorsal fiber tracts connecting the temporal and parietal cortex with the frontal cortex is still a matter of research (see, e.g., Catani, Jones, & ffytche, 2005; Makris et al., 2005). Figure 15 very roughly symbolizes SLF III and/or the arcuate fasciculus, where lower FA was found in stutterers as compared with controls. SLF III runs horizontally up to the angular gyrus, but this region seems to be more involved in the association of language with visual processing during reading and writing. For more details, see Fig. 5 in Catani & Mesulam (2008), Fig. 8 in Frey et al. (2008), Fig. 1 in Friederici (2012), Fig. 6 in Kelly et al. (2010), or Fig. 3 and 4 in Makris et al. (2005).
(return)
“Myelin formation requires cell recognition to myelinate the appropriate axon, the formation of adhesive contacts, elaboration of vast areas of cell membrane to form myelin sheets, wrapping multiple layers of membrane around axons, and the removal of cytoplasm from between the wraps of myelin to form compact stacks of lipid membrane, all of which might be influenced by signaling from electrical activity in axons. […] This would preferentially myelinate axons that are electrically active and increase the speed of conduction through these functionally active circuits. This process could therefore underlie some of the changes in white matter seen in MRI studies.” (Zatorre, Fields, & Johansen-Berg, 2012, p. 8 in the PDF)
(return)
A myelin sheath around an axon accelerates impulse conduction. However, making the entire brain faster can hardly be the function of the progressing myelination from birth to adulthood—if it were so, all axons could be readily myelinated by birth, as with those fiber tracts that, so to speak, form the brain’s operating system.
Instead, growing myelination around frequently activated nerve fibers supports the automation and habituation of frequently repeated behaviors. The networks controlling these behaviors become better myelinated, which, in turn, favors the use of these networks in the future.
That makes sense from the viewpoint of evolution because, in the conditions of the wilderness, a frequently repeated behavior can hardly be wrong—wrong behaviors are immediately punished by death, pain, fear, reproductive failure, etc., and are therefore rarely repeated. Myelination thus modulates neural development according to an organism’s environmental experience and supports non-cognitive learning through repetitive practice and the development of useful routines.
Naturally, this mechanism works in humans too. When acquiring new skills, as in crafts, arts, and sports, but also in everyday behaviors, we learn through practice and the immediate experience of success or failure, ease or difficulty, whether or not a way of doing something should be repeated. Growing myelination of the neuronal networks controlling successful, and thus frequently repeated, behaviors stabilizes and automates these behaviors so that they become habits and routines.
Unfortunately, in civilization, wrong behaviors are not always immediately punished and sometimes even rewarded. If they are frequently repeated, myelination supports habituation in these cases too, for instance, of addictive behaviors. Similarly, in stuttering, secondary behaviors that seem helpful at first but are actually not (because they reinforce the misallocation of attention) become habits.
(return)