 A Theory of Stuttering
A Theory of Stuttering
Besides the question of whether the structural deficits in the left superior longitudinal fasciculus (SLF) cause stuttering (symptoms), another question is of interest: Do these deficits play any causal role in the outbreak of stuttering in childhood? If so, the deficits ought to already be detectable in young stuttering children, shortly after the onset of their stuttering.
The first study of white matter tracts in stuttering children was done by Chang et al. (2008). Participants were right-handed boys between 9 and 12 years of age, eight with persistent stuttering, seven who had recovered but had stuttered for 2–3 years, and seven normally fluent boys (control group).
Both, the stuttering and the recovered groups, showed reduced fractional anisotropy (FA) in the left SLF. However, in the recovered group, the deficit was smaller, and greater FA was found in the right SLF compared to the two other groups (see Fig. 6 in Chang et al., 2008). Even though the last-mentioned differences were not statistically significant, they still suggest that, in the recovered boys, the deficit in fiber maturation had partially settled left and partially compensated for in the right hemisphere.
I think that the recovered boys had learned to better allocate their attention when speaking, such that more perceptual and processing capacity remained for auditory feedback. If the processing and/or integration of auditory feedback partially shifted to the right hemisphere, the reason may be that attention allocation was not yet fully automated. Maybe they paid more attention to the sound or the prosody of their speech, not so much to the phonemic structure (as normal speakers, I think, automatically do). Unfortunately, we have no data on adults who recovered from childhood stuttering and don’t know if that right-shift disappears with time.
Lower FA in the SLF in 9–12-year-old stuttering children may be the result of a longstanding misallocation of attention during speech, but what about younger stuttering children, that is, shortly after the onset of the disorder? Chang and Zhu (2013), Chang et al. (2015), and Chow and Chang (2017) investigated the white matter development in children from the age of three. They compared three groups: those who later persisted in stuttering, those who later recovered, and normally fluent controls.
The stuttering children showed structural and functional abnormalities in several brain regions, among them in the connection between cortical auditory, pre-motor, and motor areas and between cortical and subcortical regions (basal ganglia, cerebellum, and others), and in the corpus callosum. First, let us look at the connection between areas of speech perception and areas of speech control—roughly, Wernicke’s and Broca’s areas: were the structural deficits in the left SLF found in adults and older children who stutter and even, to a lesser degree, in recovered children also significant in young stuttering children?
Using probabilistic tractography, Chang and Zhu (2013) did not find significant group differences in the left SLF between stuttering children and controls. Chang et al. (2015), using voxel-based diffusion tensor imaging and with more participants, found similar structural deficits in the left SLF as were previously found in adults and older children who stutter. However, these findings can still be interpreted as a consequence or concomitant of stuttering (read more).
Chow and Chang (2017) identified four clusters with group differences in FA in the arcuate fasciculus; see Clusters 1 and 2 in Figure 1 and Clusters 5 and 6 in Figure 2 in the paper. The first two clusters seem to point to the onset of childhood stuttering, since FA was lower in both stuttering groups (persistent and recovered) compared to controls is present even in the youngest children. This may reflect a delayed development of audiomotor integration even before stuttering onset. The other two clusters (5 and 6) are probably not related to the outbreak of stuttering, but reflect developmental differences between those children who persist in stuttering and those who recover (read more).
Summarizing, we can say that the onset of childhood stuttering seems to be related to certain structural deficits in the left SLF/arcuate fasciculus. This may reflect delayed fiber maturation prior to stuttering onset due to a reduced involvement of sensory feedback in motor control (see my comment to Lazzari et al., 2024). This in turn may result from an unstable, immature attention system (e.g., delayed functional separation of the attention networks from the default mode notwork; see Chang et al., 2018) and/or from an auditory processing deficit (see Section 2.8.2).
Reduced FA in the left SLF in young children probably reflects a risk of stuttering, but I don’t believe that the underlying structural deficits (delayed fiber maturation) immediately cause stuttering symptoms.
Another finding may be closer related to the outbreak of the disorder: Chang and Zhu (2013) found that stuttering children, as a group, had a structural deficit (reduced FA) in a tract interconnecting the left pars opercularis with the posterior temporal region through a ventral tract through the extreme capsule fiber system (ECFS). Chang et al. (2015) also found reduced FA in the left extreme capsule in stuttering children, with anisotropy values being negatively correlated with stuttering severity (read more).
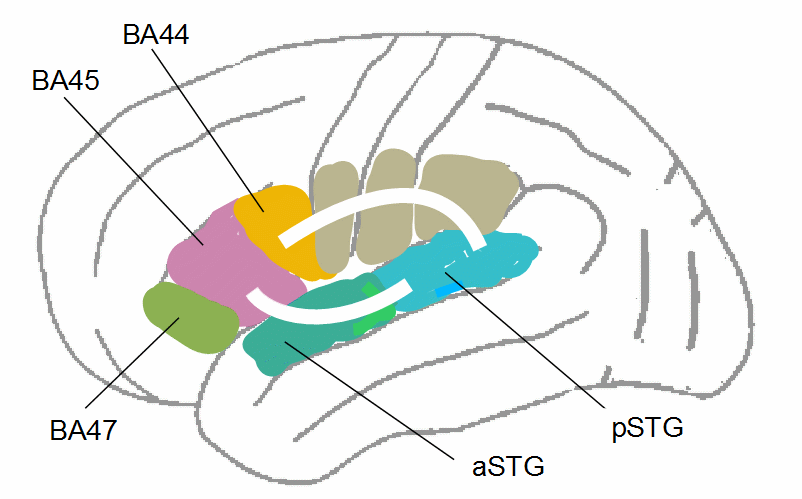
Figure 16: Charting of the SLF (as in Fig. 15) and the tract through the extreme capsule fiber system (ECFS) on the left brain hemisphere (for more details, compare Fig. 8 in Frey et al., 2008, Fig. 1 in Friederici, 2012, or Fig. 6 in Kelly et al., 2010). aSTG/pSTG = anterior/posterior superior temporal gyrus.
A structural deficit in the ECFS was not found in adults or in older children who stutter (sue, e.g., Cai et al., 2014b; Kronfeld-Duenias et al., 2016). Possibly, these deficits exist only around the onset of childhood stuttering and gradually disappear in further development not only in those who recover, but also in persistent stutterers. Chow and Chang (2017) do not report group differences in the ECFS in young children; hence, further research may clarify this issue.
The most interesting finding in the study by Chow and Chang (2017), in my view, is Cluster 4 in the splenium of the corpus callosum (Fig. 1 in the paper). The diagram shows lower FA in the persistent group compared to recovered children and controls (both range equally in this cluster). The difference is already significant among the youngest children, and there is not much overlap between the persistent group, on the one hand, and the recovered group and control group, on the other hand. This difference in FA can hardly be a consequence of stuttering.
The affected fibers of the splenium probably connect bilateral temporal regions (comp. Kuvazeva, 2013), and the lower FA in the persistent group may reflect a less effective ‘labor division’ between the brain hemispheres in auditory processing. This assumption is supported by many findings suggesting an auditory processing deficit in persistent stuttering; see Section 2.8.2 (read more).
A further brain region where FA differed between the persistent group, on the one hand, and the recovered and control group, on the other hand, is the thalamic radiation (Clusters 8–10, Fig. 2 in Chow & Chang, 2017). Here, however, the FA values of the persistent group, even for the youngest children, are often above those of the other groups and stagnate or decrease with age. The affected fibers connect the thalamus with motor and prefrontal regions, and higher FA values may indicate a more intensive interaction of the thalamic attention system (Wimmer et al., 2015) with cognition and motor control. Higher FA in this brain region in children who persist in stuttering may reflect an attentional imbalance in favor of planned, internally initiated action at the expense of perception (see Section 2.8.1).
However, the scatter plots (upper row in Fig. 2 in Chang et al., 2015) show that, in the left inferior frontal gyrus (BA44) and in the left middle temporal gyrus, differences in FA were smallest among the youngest children and became greater with age, that is, with the duration of stuttering. And there were many more regions in which the group difference in FA (stutterers < controls) was correlated with age (see Table 3 in the paper).
A significant difference between the youngest children of both groups is only in fibers beneath the motor cortex. However, this difference is because some non-stuttering children (8 out of 40) had values higher than the highest value of any stuttering child, and some stuttering children (6 out of 37) had values lower than the lowest value of any non-stuttering child, whereas the majority in both groups ranged similarly.
Apart from the six children with very low FA values beneath the motor cortex, the youngest stuttering children hardly differed from their age-matched normally fluent peers in the left SLF. The differences tend to become greater with age and (probably) the duration of stuttering. This does not suggest that structural deficits in the SLF are the cause of stuttering.
(return)
In Cluster 1 in the frontoparietal region, FA was lower on average in both stuttering groups (those who later persisted and those who later recovered) than in the control group, and lower in the recovered group than in the persistent group. The difference was also present in the youngest children, suggesting that the lower FA in this cluster was related to the onset of stuttering but not to persistence or recovery. Possibly, the affected fibers are involved in the integration of auditory feedback.
Cluster 2 is located in the posterior temporal region. Similar to Cluster 1, average FA was lower in both stuttering groups than in the control group, and lower in the recovered group than in the persistent group. The controls and the recovered group showed a slight decrease in FA with age in this cluster (which may be normal development), but the persistent group did not.
To explain this anomalous development, one may speculate that these fibers are involved in the detection of speech errors and, therefore, also in the causation of those invalid error signals that I assume to evoke stuttering. The number of true speech errors might decrease in normally developing children and in those who have recovered from stuttering, but not so the number of invalid error signals in those who persist in stuttering.
FA in Cluster 5 and 6 seems to play no role in the outbreak of childhood stuttering, since the youngest children’s values are similar in all groups. The development of FA in the two clusters indicates a growing difference between the recovering group and the persistent group: an increase in FA with age in the former, a decrease in the latter.
(return)
A negative correlation between FA and stuttering severity was also found in other brain regions where FA was reduced compared to controls (see Table 4 in Chang et al., 2015). However, these results were driven to a large extent by three of the most severe cases (all boys) among the participants. After these cases were excluded from analysis, only the left extreme capsule and the left supramarginal gyrus were significantly negative correlated with stuttering severity. The left supramarginal gyrus is a sensory association area that may also be involved in the integration of auditory feedback in speech control.
Only in those two areas (left supramarginal gyrus and left extreme capsule), the negative correlation between FA and stuttering severity was equally evident in boys and girls; in the extreme capsule, the correlation was even more significant in the girls. This suggests that structural deficits in these fibers are related to the onset of childhood stuttering, since early childhood stuttering is approximately equally likely in both sexes.
Since stuttering severity (SSI) also includes secondary behaviors, stuttering frequency is the measure more closely related to the cause of the disorder. When stuttering frequency (the proportion of stuttered syllables out of the total number of syllables) instead of SSI was entered for correlation analysis with FA, and the three most severely stuttering boys were included, only 7 instead of 24 clusters showed significant negative correlation with FA values (bold in Table 4 in Chang et al. (2015)). From these seven clusters, two were located in the cerebellum, two in the left SLF, and three in fiber tracts running through the extreme capsule, two of them in the left hemisphere. This also suggests an important role of those fibers in childhood stuttering. For the role of the cerebellum, see Section 2.2.
(return)
Ho Ming Chow, Siyan Liu, Nan Bernstein Ratner, and Allen Braun found reduced FA in the corpus callosum splenium in adults who stutter and a strong relation between FA in the splenium and stuttering severity (“Multifocal white matter abnormalities in people who stutter”, unpublished diffusion tensor imaging study; results were presented at the 2014 ASHA Convention).
(return)