 A Theory of Stuttering
A Theory of Stuttering
This section specifies my two-components model of stuttering (see also Section8nbsp;2.2.1), according to which a stuttering event has an ‘impelling’ component representing the person’s will to speak, and an ‘inhibiting’ component interrupting speech against the person’s will. In the original version of the model (see Figure 17), the basal ganglia were part of the first component, and the cerebellum was part of the second. This view was influenced by the ‘dual premotor model’ proposed by Goldberg (1985) and applied to stuttering by Alm (2006). However, the view that basal ganglia and cerebellum are separate and interact only via the cerebral cortex is obsolete.
Studies with animals and humans revealed that cerebellum and basal ganglia interact at the subcortical level and together form an integral network (Bostan & Strick, 2018; Wang et al., 2020). Thus, I had to update the model. I maintain that stuttering is caused by invalid error signals—“invalid” in the sense that no speech error happened—and that the cerebellum is critically involved in generating the error signals (see Section 2.2). However, the role of the basal ganglia (BG) is more complicated than I assumed earlier. They are not a combatant in the struggle between the two components of stuttering, but rather the battlefield.
The basal ganglia are the place in the brain where the decisions are made on what we do and what we don’t do. There are two main pathways through the BG: a so-called direct pathway that facilitates the execution of a voluntary motor action, and a so-called indirect pathway that inhibits a motor action. The two pathways operate like two reins, one for Go and one for NoGo. The prevalence of one or the other pathway determines what we do or don’t do.
Both pathways are modulated by inputs from many cortical and subcortical areas, such that knowledge, experience, and inherent instincts can influence the decision in the BG. For instance, the direct pathway gets support from the nucleus accumbens, whose activity is associated with the expectation of reward, and the indirect pathway is supported by the amygdala, whose activity is associated with fear.
In addition to the direct and the indirect pathways, there is a ‘hyperdirect pathway’, so named because it connects the frontal cortex through the subthalamic nucleus (STN) with the globus pallidus pars interna (GPi), the main output structure of the motor part of the BG, without involving the striatum, the usual BG input structure. The hyperdirect pathway was found in studies that used Go/NoGo tasks; it serves for global (non-selective) response inhibition and enables the volitional stopping of an already initiated action, e.g., if the initiation was premature or erroneous (Wiecki & Frank, 2013).
Neef et al. (2016) proposed that the hyperdirect pathway may be involved in stuttering, and Neef et al. (2017) write that “stuttering might be caused by an overly active global response suppression mechanism […] the stopping of an ongoing speech motor program and/or the selection of a succeeding speech motor program might fail.” A similar hypothesis was proposed by Orpella et al. (2024), namely, that stuttering events result from a “freeze” response (read more).
In my view, this hypothesis is not convincing, for three reasons. First, global response inhibition, as it takes place in Go/NoGo tasks, is controlled by the will; stuttering, by contrast, occurs totally against the person’s will. Second, an overly active global response suppression mechanism (as hypothesized by Neef and colleagues) would hardly affect speaking alone—or one has to explain why it is overly active only when speaking.
Third, the hypothesis doesn’t include the cerebellum, which obviously plays a crucial role in stuttering. The overactivity of right BA44 in stuttering observed by Neef and colleagus may reflect the attempt to overcome the inhibition of speech; it would then not be related to the hyperdirect pathway but to the direct pathway.
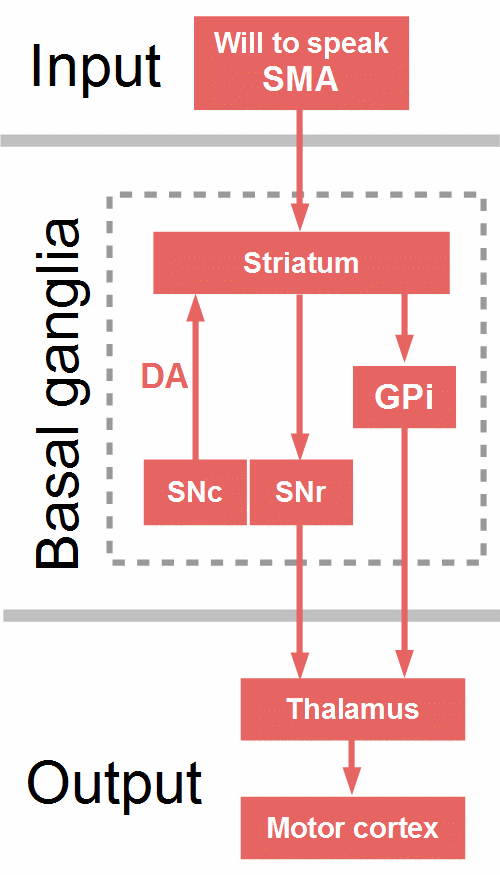 Figure 19: Direct pathway. Arrows symbolize only the direction of the projections. DA = dopamine, GPi = globus pallidus pars interna, SMA = supplementary motor area, SNc = substantia nigra compacta, SNr = substantia nigra reticulata.
Figure 19: Direct pathway. Arrows symbolize only the direction of the projections. DA = dopamine, GPi = globus pallidus pars interna, SMA = supplementary motor area, SNc = substantia nigra compacta, SNr = substantia nigra reticulata.
Figure 19 shows the direct pathway through the BG. The frontal cortex, particularly the SMA, excites ‘Go neurons’, specific medium spiny neurons. in the dorsal striatum, which inhibit GPi and SNr, the output structures of the BG. Since they themselves have inhibitory projections to the thalamus, the result is a disinhibition, that is, an excitation of the thalamus, which in turn excites the motor cortex and facilitates the execution of the selected motor program (Bostan & Strick, 2018; Wiecki & Frank, 2013).
The direct pathway is reinforced by dopaminergic projections from the substantia nigra compacta (SNc) and the nucleus accumbens (part of the ventral striatum, not depicted in Fig. 19) to the dorsal striatum. Through D1 receptors, dopamine further excites active Go cells in the striatum, and through D2 receptors, dopamine inhibits NoGo cells (Wiecki & Frank, 2013). So, dopamine supports the drive of habitual, automatic, promising behaviors, the impact of motivation and expected success or reward.
I think the role of dopamine in stuttering is ambiguous: it supports fluent speech, but when speech flow is interrupted for no clear reason, high dopamine reception in the striatum drives effort, tension, and struggle behavior, which increases the severity esp. of tonic stuttering. Studies have shown that the nucleus accumbens in the right hemisphere is larger in adults who stutter than in controls (Neef et al., 2018; Montag et al., 2019). Furthermore, Watkins et al. (2008) and Metzger et al. (2018) found a positive correlation between substantia nigra activity and stuttering severity, in the latter case in a non-speech task.
The BG not only are involved in motor control but also in attention regulation, not least because voluntary actions are always associated with selective (top-down) attention. A generally high dopamine level in the striatum might support focused attention and reduce distractibility, but also hamper attention shifting, and attention distribution. This corresponds to behavioral findings showing that stuttering children and adults performed poorer on average than controls in Go/NoGo and dual tasks (see Section 2.8.1). This is no BG dysfunction, but a personality trait. A strong propensity for goal-directed activity and focused attention is advantageous in not a few jobs, but it may increase the risk of stuttering.
In contrast to the direct pathway, which facilitates and ‘releases’ voluntary movements, the basic function of the indirect pathway is to selectively inhibit concurrent unwanted, countering movements. For instance, we cannot concurrently stretch and flex a limb. A further function of the indirect pathway is the global inhibition of premature, erroneous, or potentially dangerous actions. Global inhibition can be elicited volitionally, that is, by input from the frontal cortex to the STN (hyperdirect pathway, see above), but I think it can also be triggered involuntarily and even against the will, namely by input from subcortical structures such as the cerebellum, the amygdala, or the periaqueductal gray (the activity of the latter is associated with fury). Studies in humans and animals have shown that these structures are interconnected with the STN (Accolla et al., 2016; Hachem-Delaunay et al., 2015; Smith, Hazrati, & Parent, 1990).
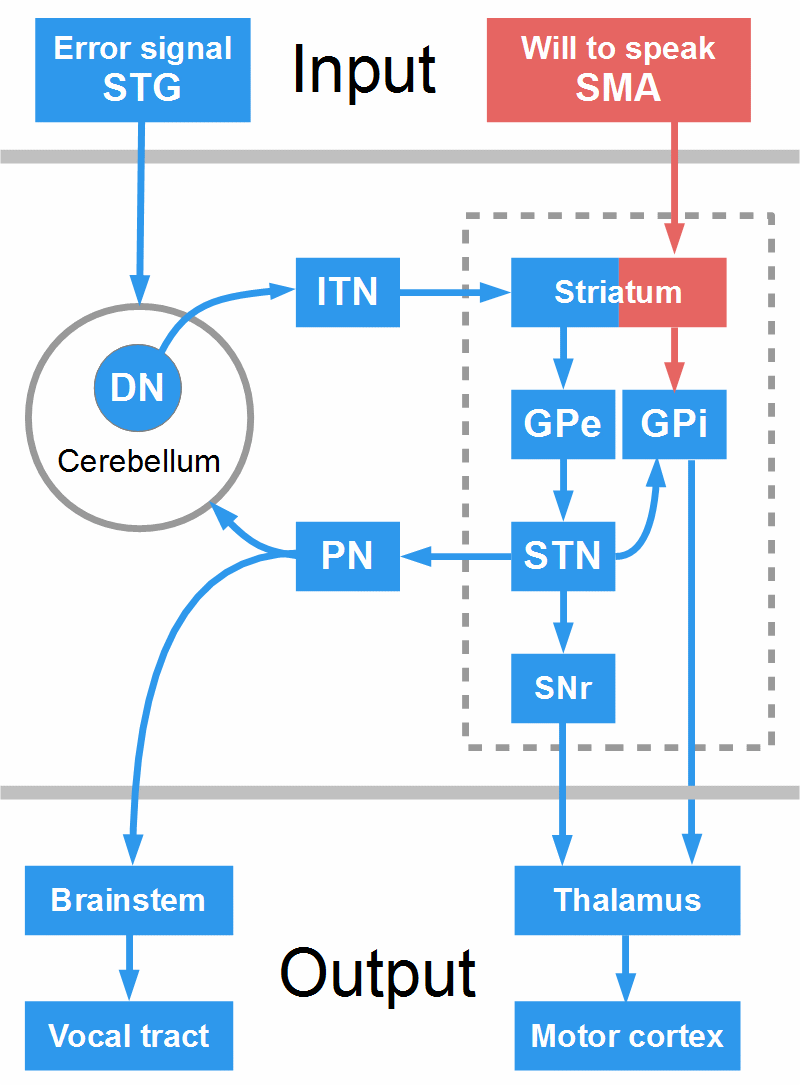 Figure 20: Indirect pathway and the cerebellum-BG loop. Arrows symbolize only the direction of the projections. DN = dentate nucleus, GPe = globus pallidus pars externa, GPi = globus pallidus pars interna, ITN = intralaminar thalamic nuclei, PN = pontine nuclei, SMA = supplementary motor area, SNr = substantia nigra reticulata, STG = superior temporal gyrus, STN = subthalamic nucleus. The figure does not respect spatial relationships; for instance, the ITN, of course, are part of the thalamus.
Figure 20: Indirect pathway and the cerebellum-BG loop. Arrows symbolize only the direction of the projections. DN = dentate nucleus, GPe = globus pallidus pars externa, GPi = globus pallidus pars interna, ITN = intralaminar thalamic nuclei, PN = pontine nuclei, SMA = supplementary motor area, SNr = substantia nigra reticulata, STG = superior temporal gyrus, STN = subthalamic nucleus. The figure does not respect spatial relationships; for instance, the ITN, of course, are part of the thalamus.
To inhibit an acion, the cortex sends excitatory projections to NoGo cells in the dorsal striatum (this is not depicted in Fig. 20, but imagine the red boxes and arrows in the figure were blue). The striatal NoGo cells send inhibitory projections to the GPe, and the GPe has inhibitory projections to the STN; thus the inhibition of the GPe disinhibits, that is, activates the STN. Now, the STN, through excitatory projections, activates GPi and SNr, the BG output structures, which inhibit thalamus and motor cortex.
Figure 20 shows what may happen in stuttering. The cortex sends excitatory projections to Go cells in the striatum (representing the will to speak) and, just as in the direct pathway, these cells try to inhibit the GPi (this is symbolized by the red boxes and arrows). But now, the loop between BG and cerebellum comes into play. This loop interconnects the monitoring system (STG, cerebellum, thalamus) with the ‘executive system’ (SMA, BG, thalamus, motor cortex). During the execution of a motor sequence such as speech, the STN permanently ‘asks’ the cerebellum if each element of the sequence (each word, syllable, or phrase) has been produced properly.
The cerebellum, or a loop between cerebellum and STG, compares auditory predictions with auditory feedback. If a mismatch is detected, input from the cerebellum excites NoGo cells in the striatum. This activates the indirect pathway that takes over: As depicted in Fig. 20, the GPe is inhibited, which activates the STN that excites GPi and SNr, and they inhibit thalamus and motor cortex.
This view is consistent with Metzger et al, (2018) who observed an increased functional coupling between GPe and cortex, suggesting “an excessive activity of the indirect pathway and, thus, an increased inhibitory action on the cortex during stuttering, which is in line with previous reports of the fluency-enhancing effect of D2 receptor blockers” (p. 177).
The empirical basis for the proposed scenario comes from studies in mammals and humans in which BG and cerebellum were found to form an integral network (for an overview, see Bostan & Strick, 2018). The STN is the source of a dense disynaptic projection to the cerebellar cortex (lobule VI, crus I/II) via the pontine nuclei, and the dentate nucleus in the cerebellum is the source of a dense disynaptic projection to the striatum via the intralaminar thalamic nuclei (Bostan & Strick, 2018; Wang et al., 2020). The cerebellar output targets NoGo neurons in the striatum; that is, it preferentially influences the indirect pathway (Bostan & Strick, 2018).
Not a few studies have shown that the cerebellum is involved in the detection and correction of motor errors on the basis of sensory feedback (see Section 2.2). For instance, Zheng et al. (2013) identified a brain network that appeared to encode an error signal in response to distorted auditory feedback during articulation. Seidler, Noll, and Chintalapati (2006) found BG activation in a task involving error-based sensorimotor adaptation. Bostan and Strick (2018) have assumed that the pathway from the cerebellum to the striatum underlies this activity.
One may ask: Is it likely that cerebellar output interferes so severely with the process in the BG? An instance of cerebellar impact on the BG is dystonia. Similar to stuttering, dystonia has often been considered a BG disorder, but growing evidence suggests that the cerebellum is involved. In animal models, dystonic postures were evoked through abnormal cerebellar output and abolished by silencing or lesioning the cerebellum (Bostan & Strick, 2013; Tewari, Fremont, & Khodakhah, 2017). However, whereas dystonia seems to be caused by cerebellar dysfunction, the cerebellum does its job in stutterers when is responds to error signals and stops speaking.
In stuttering, inhibition of the motor cortex is possibly not the crucial effect of the STN activation. Inhibition of the motor cortex would only mean that muscles were no longer activated. But overt stuttering symptoms imply muscle activity; for instance, the contraction of antagonistic muscles to stop the current movement or the prolongation of the current muscle contraction to stop the transition to the next movement. Possibly, this may be triggered via a pathway from the STN to the brainstem.
As mentioned above, the projection from the STN to the cerebellum is mediated by the pontine nuclei. According to Marien (in Manto et al., 2012), two distinct neural networks control speech sound production: a linguistic network (involving Broca’s area, SMA, anterior insula, BG, and cerebellum) and a phylogenetically ancient motor pathway for vocalization, which encompasses, among others, BG and pons and projects to cranial nerve motor nuclei in the lower brainstem. These nuclei innervate the vocal tract musculature.
This pathway from the BG to the brainstem may inhibit or interrupt speech flow and evoke stuttering. This would also account for the stutterer’s subjective experience that an unknown power, against the person’s will and stronger than the will, for reasons inaccessible to consciousness, suddenly interrupts speaking.
As already mentioned, I think that the overactivation of the STN-cerebellum loop following an invalid error signal triggers a global inhibition. According to Wiecki & Frank (2013), the STN, through excitatory projections to the SNr, enhances the threshold for any action, independent of action modality. The assumption that global response inhibition precedes overt stuttering is consistent with the fact that a stutter block becomes weaker after a short time, such that the speaker can continue (until the next block).
This corresponds to the course of global response inhibition: when the STN is over-activated, it also sends excitatory projections to the GPe (this is not depicted in Fig. 20). Since the GPe has inhibitory projections to the STN, the excitation of the GPe, after a delay, inhibits the STN, such that the global NoGo signal becomes weaker and disappears. Then, the direct pathway takes over and enables the execution of the selected motor program (Frank, 2006; Wiecki & Frank, 2013).
Orpella et al. (2024) hypothesized that stuttering—at least if following the anticipation of stuttering—is due to reactive inhibitory control, which “refers to an automatic and rapid response triggered by an external cue to halt a planned action. Reactive inhibitory control is typically tested using stop signal tasks” (p. 434). The authors assume that, in stutterers, reactive inhibitory control may be “triggered by an external cue that signals that overt stuttering is imminent” (p. 434).
The problem here is the same as with the above-mentioned proposal by Neef et al. (2016). Reactive inhibition is initiated by the person’s will, but the interruption of speech when stuttering happens against the person’s will. The authors tried to get around that problem by presenting a cue prior to the go signal (for speaking); the cue “informed participants of the imminent requirement to produce the word” (p. 434). This cue, the authors assume, “will act as an implicit no-go signal because of the stutterer’s learned association between stuttering and its negative consequences” (p. 435).
However, it’s questionable if the cue acted as an implicit no-go signal, since the participants certainly knew that their stuttering would have no negative consequences in the specific situation; on the contrary, it was desired. I rather think that the participants understood the cue as a signal to make an effort to get the word out when the go signal appears, despite the anticipation of stuttering.
The increased beta power for stuttered versus fluent trials in the right preSMA following the cue, which the authors have interpreted as reflecting the activity of the action-stopping network, may rather reflect the tension of the will to speak the word despite the anticipation of effort to get it out. The preSMA is not only involved in stopping but also in initiating actions, particularly if initiating an action is associated with cognitive load and decision-making (Cummine et al., 2017; Zhang, Ide, & Li, 2012).
(return)